Sandy beaches, traditionally regarded unreactive due to their low carbon content, were found to be hotspots of carbon-fueled reactions that can decrease nitrate contamination in nearshore waters. During waves and tides, seawater delivers particulate organic carbon into the sands that are distributed in complex patterns and fuels reactions.
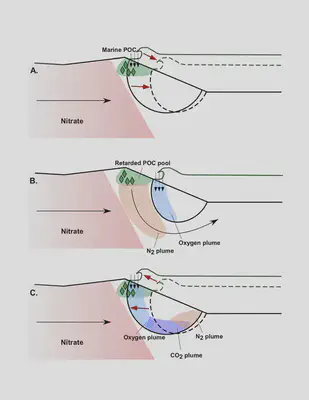
In this work, we modeled the complex mixing patterns of terrestrial groundwater and seawater, to understand hydrologic controls on reactions between particulate carbon and other solutes (e.g., oxygen, nitrate).
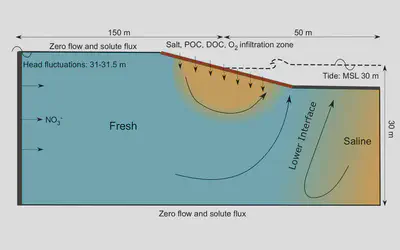
This project, for the first time, provided sensitivity analyses for variable-density systems that involve reactions that depend on both particulate and solute reactants. Such analyses are useful as modeling water quality often involves density differences or solid phases of contaminants.